Understanding Rett through the Power of Artificial Intelligence

Earlier this year RSRT announced a $1.1 million award to Dina Katabi and her team at Emerald Innovations to expand their work using the Emerald device to assess symptoms of irregular breathing, sleep disruptions, and movement in individuals with Rett in a new validation study.
The Emerald is a device that is placed in the home and assesses a person’s physiology without ever touching them. It uses artificial intelligence to identify movements, sleep, and vital signs from very low power radio waves. RSRT has previously funded a pilot study using Emerald which yielded very encouraging results.
Some of you may be thinking, “With gene replacement clinical trials starting, won’t it be plainly obvious if symptoms improve or not? Why bother trying to develop better symptom assessment tools?”
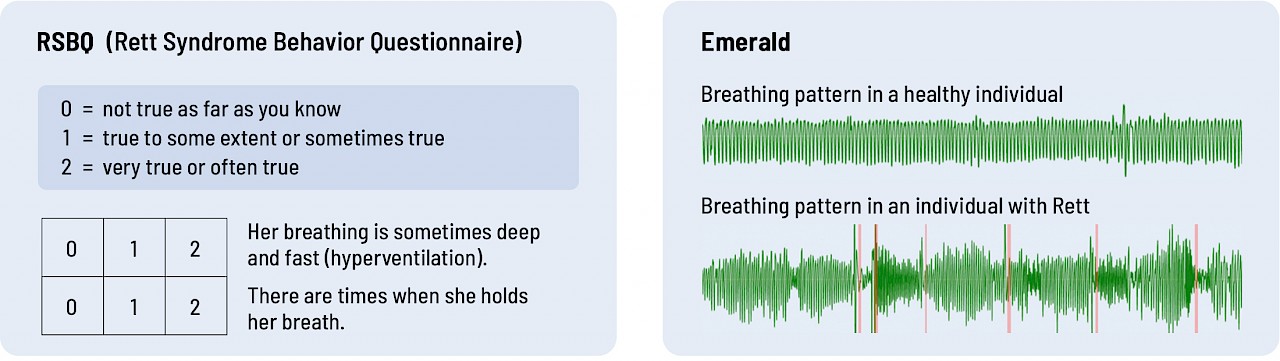
Great questions and important for me to answer. Before we get into all the reasons, let me first show you the difference between Emerald and the current standards. To date, the outcome measure for Rett clinical trials has been the parent-reported Rett Syndrome Behavior Questionnaire (RSBQ) and the Clinical Global Impression of Improvement (CGI-I).
Take a look at the graphics below. Which do you think provides more objective, quantitative data on breathing and sleep – RSBQ and CGI-I or Emerald?
Breathing
Sleep
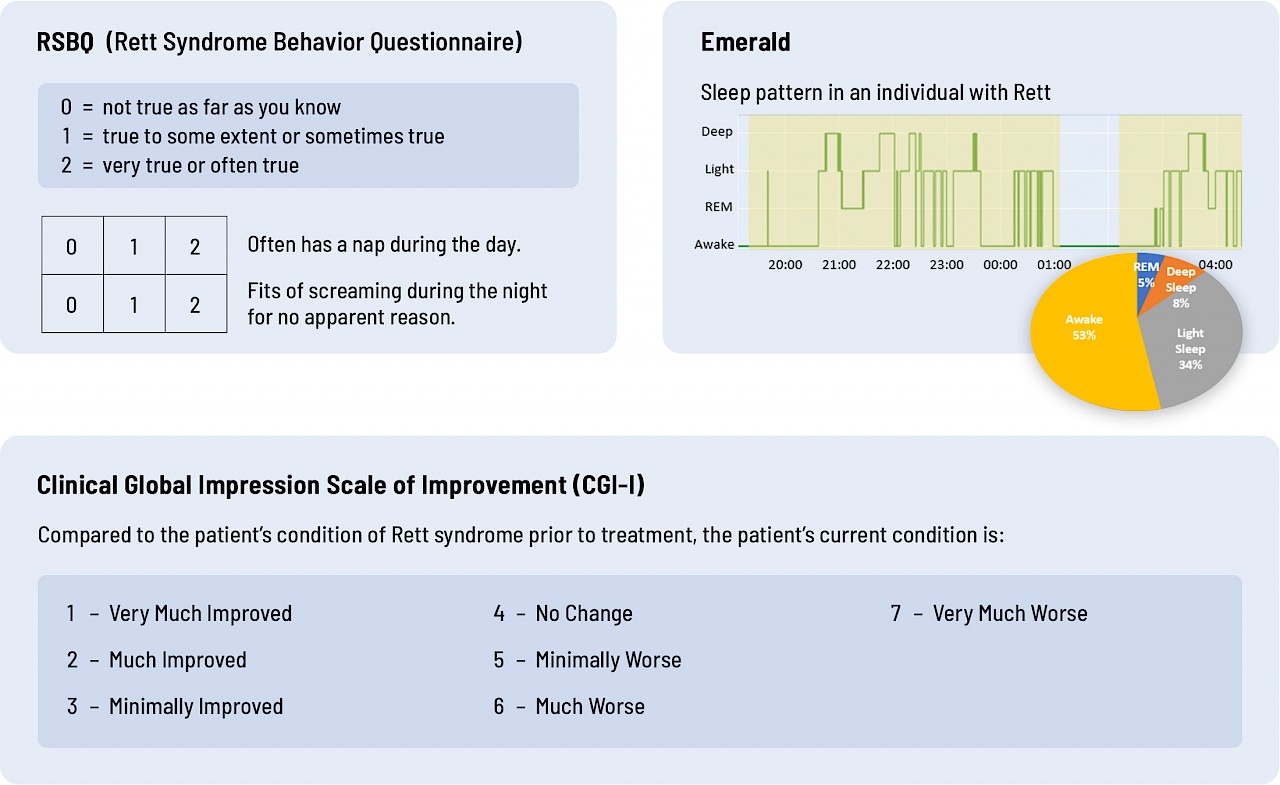
Current assessment tools are just not sufficiently sensitive or objective. You don't need to be an MD or a PhD to see that the data Emerald collected directly from an individual provides richer, more quantitative data than a questionnaire that asks parents or clinicians to interpret how their child or patient is doing.
Clinical trials would significantly benefit from assessment tools that are sensitive, accurate, and objective, and that focus on symptoms that are critical to our children’s well-being. Having tools like Emerald will help ensure that medicines being tested and approved for Rett provide meaningful improvements.
Of course, we hope that the upcoming gene replacement trials and all future genetic medicine trials will result in dramatic improvements that will be obvious to all with a simple before and after video. However, regardless of how dramatic the results are, they will take time to manifest.
Here are several reasons why having an objective assessment tool is important:
- An objective assessment tool that can pick up subtle early symptom changes will provide important clues that the medicine is working (or not working). It’s important for biopharma companies to know as soon as possible whether they are seeing results or adverse side effects with their medicine. Early signals inform safety, dosing, study duration, participant numbers, and other factors that determine how quickly a development program can be completed.
- An objective assessment tool could be used to help stratify or select patients for trials so that symptoms can be precisely measured and patients appropriately grouped for treatment and analyses.
- An objective assessment tool could help determine dosage of a therapeutic and if it needs adjusting.
- An objective assessment tool will be used beyond genetic medicines with drugs that are not targeting the root cause of the disease and where improvements may be subtle.
- The current lack of reliable symptom assessment tools is hindering biopharma companies from entering the Rett space. I have no doubt that having these tools will open the floodgates for additional biopharma companies to take on Rett therapeutic programs.
- Having objective assessment tools will allow for smaller and faster clinical trials which means development timelines will shrink — getting treatments that don’t work out of development earlier and those that do work available to the community sooner.
While biopharma companies see incredible value in having reliable assessment tools, they don’t have the time, resources, or bandwidth to develop them. It’s therefore crucial that RSRT addresses this gap to support Rett development programs.
The validation study will be recruiting patients from the Rett clinics at Boston Children’s Hospital and Rush University in Chicago. We will share more information as the study prepares to recruit.
I thank all of our families and supporters who make this study possible and join us in our mission to cure Rett.
Resources
Award Press Release
Emerald Ted Talk
MIT CSAIL Talk
Emerald Innovations


