Rett and Pharma... What's the Attraction?

I often come across statements to this effect, “The pharmaceutical industry is not interested in pursuing drug development for Rett Syndrome because the disorder is rare and companies won’t make any money.”
And yet, I am fielding emails, calls and in-person meetings with industry executives almost on a daily basis. From my perspective, Rett is clearly on the industry’s collective radar. This goes for both large pharmaceutical companies and smaller biotech firms, but why?
Industry is flocking to rare diseases and here are some of the reasons why:
- The biology of rare diseases is often better understood than that of common diseases. This is certainly the case with Rett because it is caused by a mutation in a single gene that has already been pinpointed.
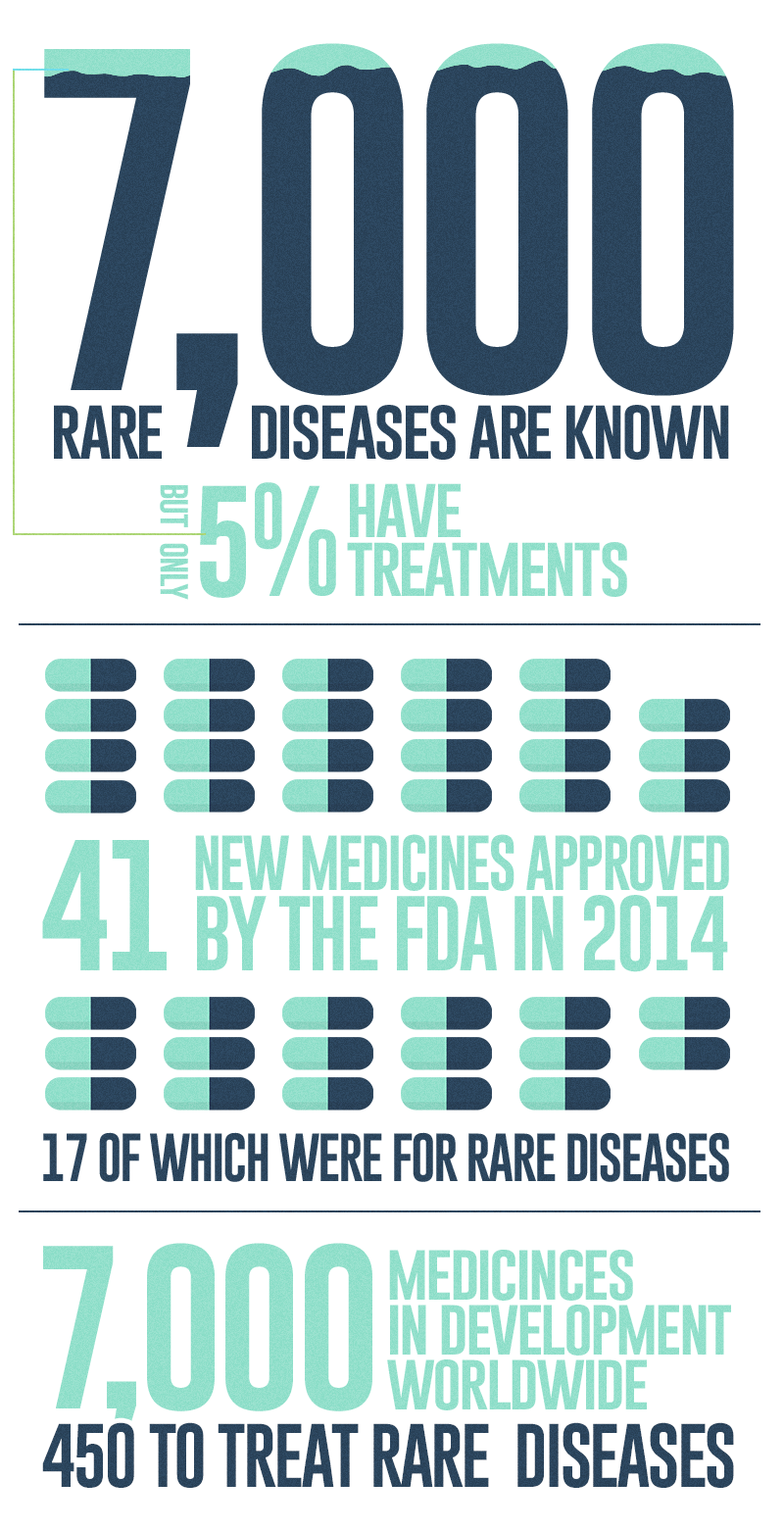
- The US Orphan Drug Act, created to facilitate the development of drugs for rare diseases, provides companies with tax credits, funding grants for clinical trials, a waiver of FDA fees and 7-year market exclusivity.
- Rare disease drugs can demand hefty price tags. Annual costs of $100K to $500K are not unusual.
- Rare disease drugs have reduced marketing costs and often increased reimbursement possibilities.
- Clinical trials are much smaller therefore cheaper, and enthusiastic patient population often makes for easier trial recruitment.
- Drugs designed to treat rare disorders sometimes prove to be beneficial for a broader patient group.
Companies like Genzyme, Shire, Vertex, Alexion, BioMarin, Celgene and Aegerion just to name a few, have firmly established a successful business model for rare disease drug development.
This paradigm shift is good news for Rett. In addition to the above perks Rett offers a unique advantage that has not gone unnoticed by industry executives: reversibility!
While it’s extremely gratifying to witness this activity we must not let upon the intensity with which we support and drive basic science and clinical research. Industry’s interest in the therapeutic approaches that RSRT has been pursuing (activating the silent MECP2, modifier genes, downstream targets such as NMDA pathways, gene therapy) validates our research strategy. Our research has fueled a rich pipeline of potential drug targets and it’s imperative to keep that pipeline flowing.